
For instance, you could try to put molecules on the surface of a nanoparticle so it would make a defined number of “bonds” to other nanoparticles:ĭirectional assembly of asymmetrically functionalized AuNPs into (A, B) cat paw, (C, D) satellite, and (E, F) dendrimer-like structures. The heterogeneity of nanoparticles means it is hard to get them to assemble into well-defined structures. But nanoparticles can be made into spheres, rods, stars, and many other shapes. All atoms are approximately shaped like a sphere, with a very tiny nucleus (diameter 1×10 -15 m) enrobed in a cloud of electrons (overall diameter 1×10 -10 m). Atoms in a sample of a pure element are chemically identical, but individual nanoparticles in a batch can vary a little bit in size, shape, and surface characteristics.

Of course, nanoparticles are made of atoms! Individual atoms are about 1x 10 -10 m in diameter, but nanoparticles are usually 10 to 1000 times larger. Laid out, there are some big differences between individual atoms and Regardless of how a “periodic table” of nanoparticles would be Nanotechnologists have tried to come up with a “periodic table of nanoparticles” – here is one simplified example:Īlthough it doesn’t look much like the classic Periodic Table, this diagram was recently published as a sort of “periodic table of nanoparticles” showing how molecules might self-assemble into nanoparticles of different types and shapes (image from Packwood & Hitosugi 2018, 2 courtesy of CC By 4.0 license) It makes beautiful sense that elements with the same physical and chemical properties have the same number of valence electrons, and ended up organized together on the periodic table. Germanium was not known in Mendeleev’s time,īut he knew of the others, and therefore predicted that germanium ought toĮxist, have the atomic number we know now, and the reactivity we know now!Ĭoming back to the periodic table, the amazing thing is that 150 years ago, no one knew electrons existed at all, let alone that they had particular energy levels! But the reactivity of the elements is dominated by their valence electron counts, so by organizing the elements according to their reactivity, Mendeleev anticipated this atomic feature that had not been discovered yet. Those electrons are in different orbitals) and they too make stable compounds 1 In order to appreciate how amazing this is, we need to understand a little bit about how atomic structure works.Īn example of how each carbon atom has four bonds to connect to other atoms, in this case bonding to hydrogen to form a molecule of butane (image by NEUROtiker )Īll the other elements in the column of the periodic table belowĬarbon (silicon, germanium, tin, lead) also have 4 valence electrons (although Mendeleev, the author of the periodic table as we know it, knew many chemical and physical properties of the elements, and even predicted that certain elements should exist before they were discovered (germanium, gallium). Organizing the periodic table by properties like this may seem obvious, but one of the triumphs of the table is that it relates these easily measurable properties (like boiling points, melting points, reactivity) to atomic-level properties. All of these elements are gases at room temperature and pressure, and are very unreactive hence, the common name for this column of elements is the “noble gases.” Why the Periodic Table is Amazing The last column on the right, for instance, contains helium (He), neon (Ne), argon, (Ar), krypton (Kr), xenon (Xe) and radon (Rn). If we pick a column in the periodic table, we can find that the elements in that column have similar properties. For example, carbon (C ) is number 6 in the Table that means all carbon atoms in the universe have exactly 6 protons in their nuclei. The atomic number of an element corresponds to how many protons it has in its nucleus.
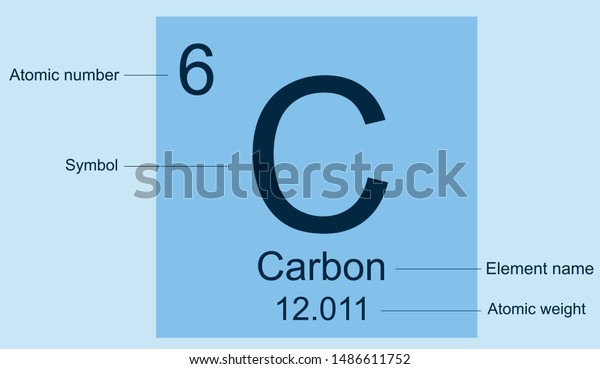
(The above picture of the periodic system is interactive - no need to download, just click on an element.A simple periodic table showing the arrangement of elements in rows and columns.
CARBON PERIODIC TABLE WITH PICTURE PDF
Now available: history of the periodic tableĬhoose elements by name, by atomic number, by symbol, by massĬlick here for the history of the periodic table.Ĭlick here to download a PDF version from that periodic table An interactive, printable extended version of the Periodic table of chemical elements of Mendeleev (who invented the periodic table).



 0 kommentar(er)
0 kommentar(er)
